News
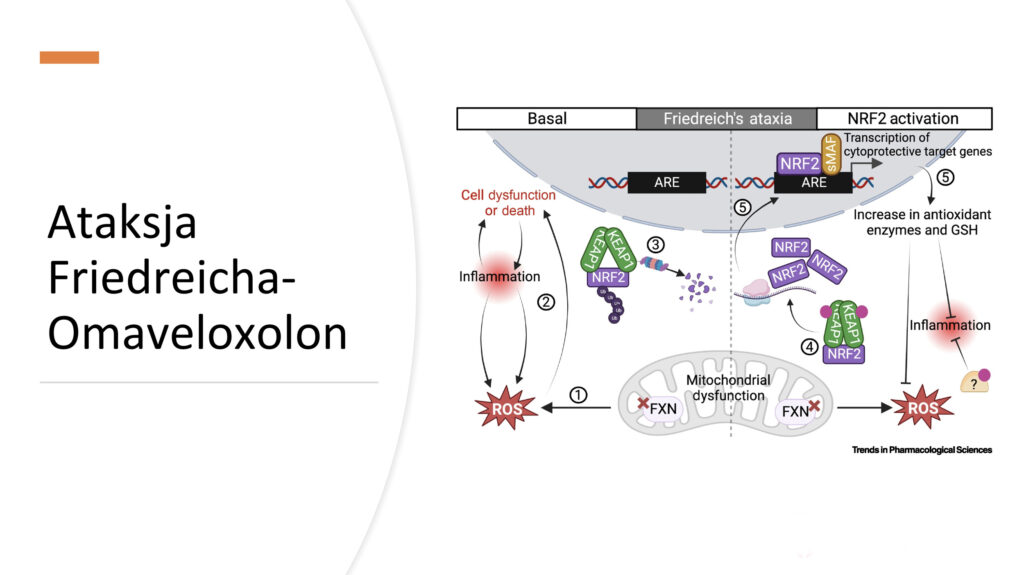
backAfter the lecture by Prof. Katarzyna Kotulska-Jóźwiak, introducing the topic of Friedreich’s ataxia, Dr. Mariusz Kwarciany presented detailed clinical studies on the treatment of FA with omaveloxolone. This is currently the first and only therapy that slows the progression of the disease

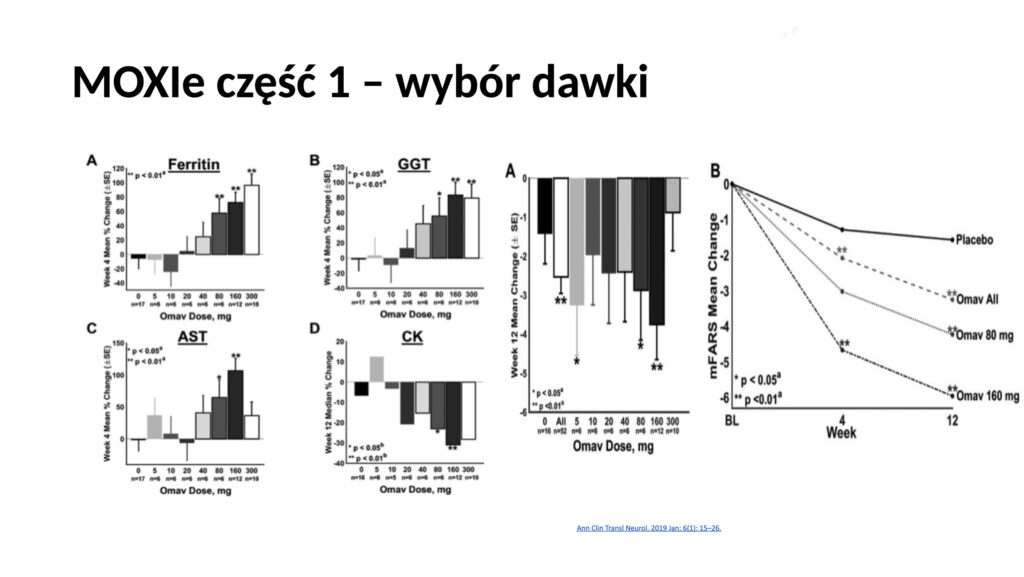
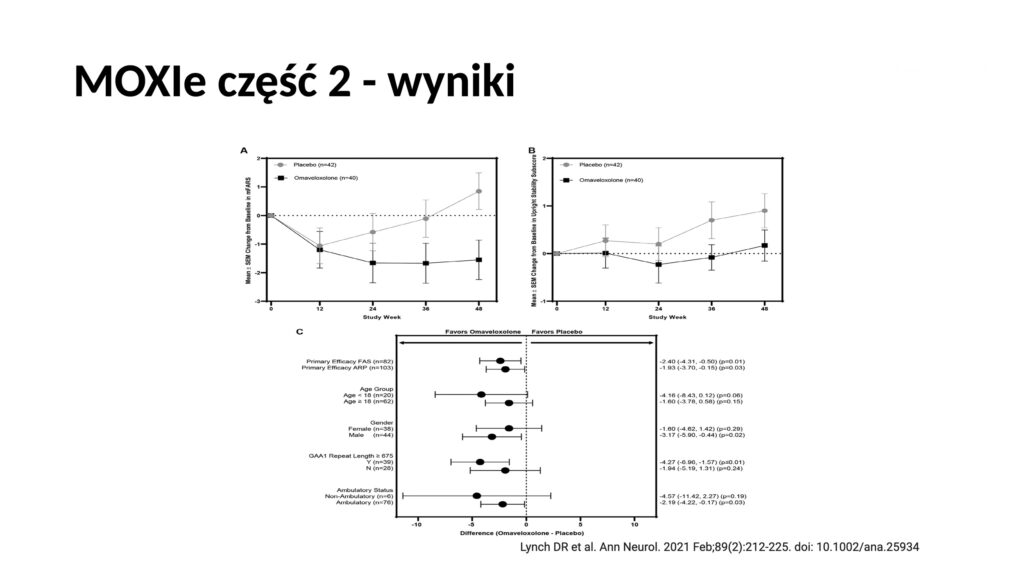
 The expert meeting was recently held at the Institute of “Pomnik Centrum Zdrowia Dziecka” in collaboration with IRSS. Dr. Mariusz Kwarciany, MD, from the Medical University of Gdańsk, presented the long history of the search for effective therapy for FA, and omaveloxolone emerged as a promising candidate after studies on animal models and patients. In clinical trials (MOXIe), this drug improved patient outcomes on the mFARS scale (which measures the severity of ataxia symptoms). A dose of 150 mg proved to be the most effective, although some patients, especially those with “Friedreich’s foot,” responded less favorably.
The expert meeting was recently held at the Institute of “Pomnik Centrum Zdrowia Dziecka” in collaboration with IRSS. Dr. Mariusz Kwarciany, MD, from the Medical University of Gdańsk, presented the long history of the search for effective therapy for FA, and omaveloxolone emerged as a promising candidate after studies on animal models and patients. In clinical trials (MOXIe), this drug improved patient outcomes on the mFARS scale (which measures the severity of ataxia symptoms). A dose of 150 mg proved to be the most effective, although some patients, especially those with “Friedreich’s foot,” responded less favorably.
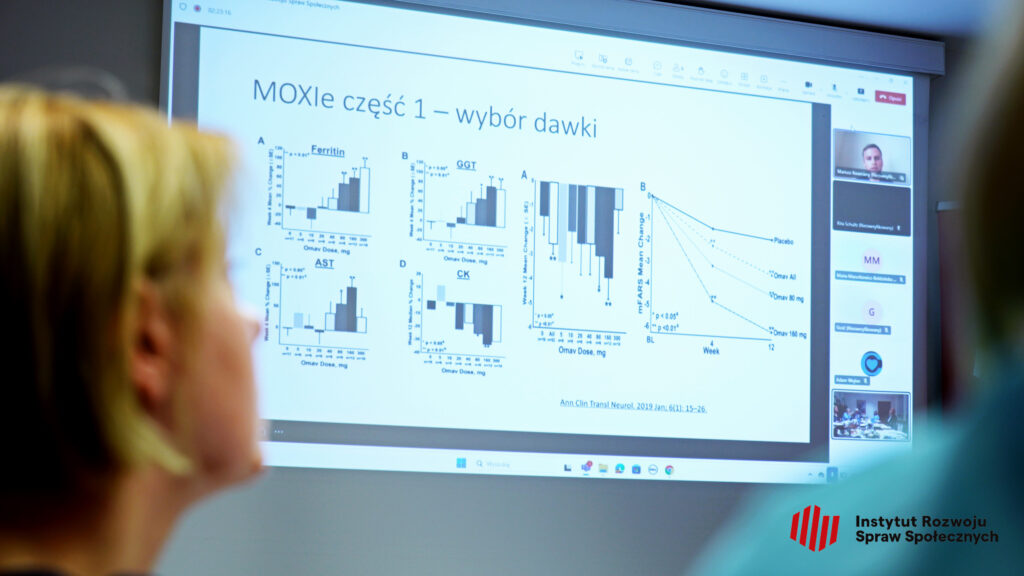
 The studies showed that starting therapy earlier led to better outcomes. Patients who began treatment earlier maintained improvement over a longer period. In summary, omaveloxolone is the first effective drug that slows the progression of FA, but further research is needed, especially concerning children and the therapy’s impact on other aspects of the disease, such as cardiomyopathy or scoliosis.
The studies showed that starting therapy earlier led to better outcomes. Patients who began treatment earlier maintained improvement over a longer period. In summary, omaveloxolone is the first effective drug that slows the progression of FA, but further research is needed, especially concerning children and the therapy’s impact on other aspects of the disease, such as cardiomyopathy or scoliosis.
Omaveloxolone was approved by the FDA in March 2023, and in February this year, it was also approved for the treatment of FA by the European Medicines Agency.
#rarediseases #irss #FA #friedreichsataxia
